Counting Atoms, Phase Changes, Covalent Bonds 10/14/17
Summary:
 |
| Covalent Bonds Link |
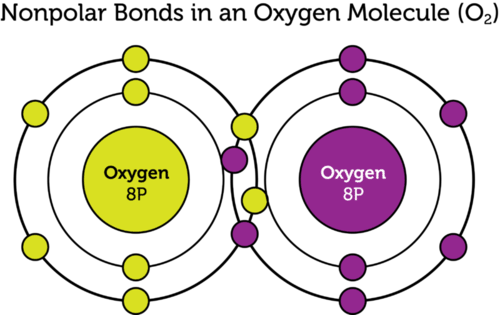
This week in science what I learned about was how to count atoms, phase changes with states of matter, and covalent bonds with atoms. What I learned about counting atoms was what all the types of numbers (in terms of what they mean and do not actual numbers) and what they mean. The first type of number I learned was the subscript, now the subscript represents how many of the element before the subject has in the molecule. The next type of number I learned was the subscript after the parenthesis, this number represents that all the amounts of each element in the parenthesis is actually whatever the answer is to multiplying each element's amount of atoms by the number after the parenthesis. For example, if you have Zn(MnO4)2 (both numbers represent subscript) this means that you have 1 Zinc atom, 2 Manganese atoms (1 x 2) and 8 oxygen atoms (4 x 2). The last type of number I learned about was the coefficient. The coefficient is the number that is the first thing in the chemical formula. It acts just like subscript after a parenthesis except it now applies to the whole chemical formula. So if you had 4CaCO3 (the 3 is subscript) this means you would have 4 calcium atoms, 4 carbon atoms, and 12 oxygen atoms (3 x 4). The last thing I learned about counting atoms is what you do when you have all three types of numbers in your chemical formula. What you do is you just treat all of them the same and allow all of them to do their job. For example, if you have 3Pb(NO3)2 (the 3 in the parenthesis is subscript as well as the 2) this means that you have 3 (3x1) lead (Pb) atoms, 6 ( 3[1x2] ) nitrogen (N) atoms, and 18 ( 3[3x2] ) oxygen (O) atoms. Then the sum of all those numbers (that aren't in the parenthesis from the sentence before) you get the total amount of atoms. What I learned about phase changes is that one of the biggest things that dictates what phase (solid, liquid, gas) the atom is in is the temperature. What I learned about covalent bonds is what they are, which is a bond that forms when atoms are sharing electrons.
S&EP 5 - Using Mathematics & Computational Thinking:
This week in science we had to use computational thinking by having to calculate how many of each element their were and how much in total atoms there were in chemical formulas. The certain variables we identified were the three types of numbers in chemical formula's. Which are the subscript, the subscript after the parenthesis, and the coefficient. We had to use mathematics such as algebra, multiplication, and addition to calculate how many atoms of each element there were and how many atoms in total.
XCC - Cause & Effect:
The cause and effect we studied this week was compound mixing to create new compounds. The cause and effect I observed was through this game we had to play in science that involved finding certain compounds and having to mix them and come out with a certain compound. Predictions I can make before this chemical reaction cause and effect is what elements will come out of the two compounds after mixing it. The prediction I can make is that the elements that are in the current compounds are the only elements that will be the in the product(s). For example if you are mixing Na2CO3 with Ca(OH)2 the only elements that can come out of it are calcium (ca), oxygen (O), hydrogen (H), carbon (C), and sodium (Na). I am correct because when mixing those 2 compounds the products are NaOH and CaCO3.
Multiplier-Learner:
This week I was a learner, I was a learner because coming into this subject I really almost had no idea how and what to do. But I wanted to know so I could be successful. So everything I wanted to know about I went ahead and asked, searched and did many things to go ahead and try to understand.
Comments
Post a Comment